Photosynthetic microalgae are of interest world-wide as potential feedstock for fuels because of their high productivity of oil, high growth rate, tolerance of diverse environmental conditions and capability to grow on non-arable land using brackish water and wastewater. However, the metabolic and regulatory mechanisms underlying their robust oil production remain elusive. A research team led by Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academyof Sciences (CAS-QIBEBT) has now reported the first spatiotemporal molecular model of the oil production process in oleaginous microalgae. This work was published in Plant Cell in 1st, April (Li, et al, Plant Cell, 2014).
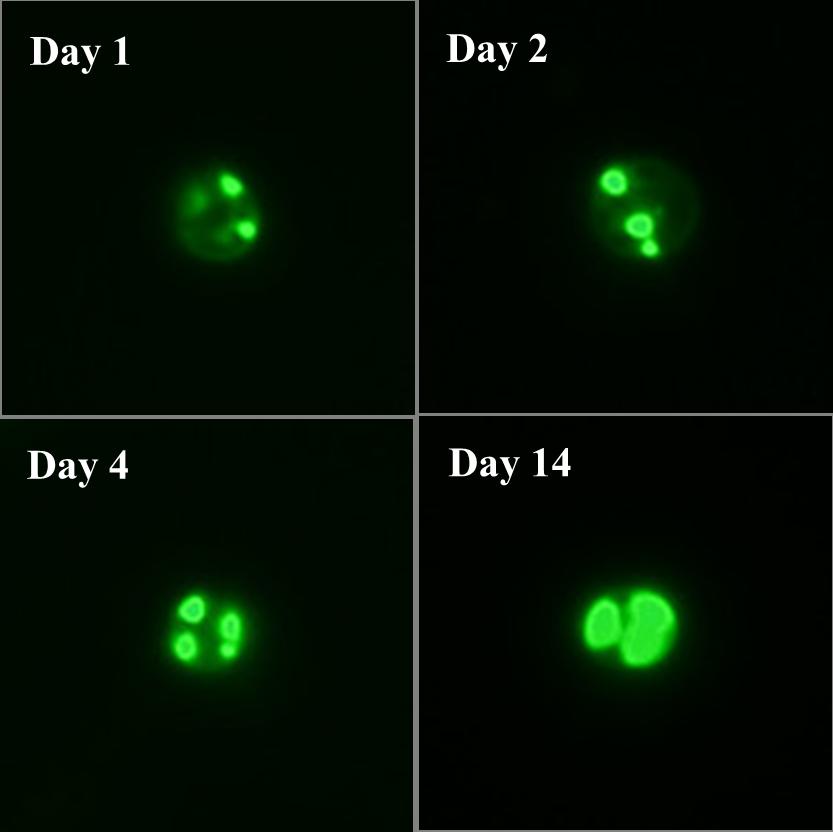
Fig. 1. Oil accumulation in microalga Nannochloropsis spp. Neutral lipids (mostly TAG) were specifically labeled by Bodipy (green fluorescence) in Nannochloropsis oceanica cells, which accumulate abundant oil under nitrogen-depletion conditions.
(The images were taken by JIA Jing)
Nannochloropsis spp. are a group of wild oleaginous microalgae that are widely distributed in the ocean and are cultivated world-wide. Previously this research team has revealed the genetic foundation and evolution of oleaginous traits in microalgae using Nannochloropsis as a model (Wang, et al, PLoS Genetics, 2014). However the molecular mechanisms underlying the robust oleaginousness remain largely unknown.
In this new work, PhD student LI Jing, Dr. WANG Dongmei and Dr. NING Kang from Single-Cell Center, CAS-QIBEBT, Dr. HAN Danxiang from Arizona State University and their colleagues simultaneously tracked the transcriptomic and lipidomic dynamics of the oleaginous microalga Nannochloropsis oceanica under nitrogen-replete (N+) and N-depleted (N-) conditions (Figure 1). At the transcript level, enhanced triacylglycerol (TAG) synthesis under N- conditions primarily involved upregulation of seven putative diacylglycerol acyltransferase (DGAT) genes and downregulation of six other DGAT genes, with a simultaneous elevation of the other Kennedy pathway genes. Under N- conditions, despite downregulation of most de novo fatty acid synthesis genes, the pathways that shunt carbon precursors from protein and carbohydrate metabolic pathways into glycerolipid synthesis were stimulated at the transcript level. In particular, the genes involved in supplying carbon precursors and energy for de novo fatty acid synthesis, including those encoding components of the pyruvate dehydrogenase complex (PDHC), glycolysis, and PDHC bypass, and suites of specific transporters, were substantially upregulated under N- conditions, resulting in increased overall TAG production. This is the first temporal and spatial regulation model of oil accumulation in microalgae, and therefore provides a basis for improving our understanding of TAG synthesis in microalgae and will also enable more rational genetic engineering of TAG production.
This first molecular model of microalgal oil-production process also unraveled the distinction in transcriptional regulation in oil synthesis between wide oleaginous microalgae (e.g., Nannochloropsis spp.) and nonoleaginous Chlamydomonas reinhardtii. The greatly increased number of copies of DGAT genes and the much higher degree of transcript levels under both under N+ and N- conditions might have conferred the ability of Nannochloropsis spp. to produce considerably larger amounts of TAG under stress.
This work was led by Prof. XU Jian from Single-Cell Center of CAS-QIBEBT and Prof. HU Qiang from Center for Microalgal BioEnergy and Biotechnology in Institute of Hydrobiology, CAS.