Probiotics claim to host a slew of health benefits for a person. Rapid expansion of the probiotics industry demands fast, sensitive, comprehensive and low-cost strategies for quality assessment.
A research team led by the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) has developed a new approach called Single-Cell Identification, Viability & Vitality tests, and Source-tracking (SCIVVS), based on the ramanome concept, to assess the contents and quality of various probiotics efficiently and cost-effectively.
The results were published on May 25 in iMeta.
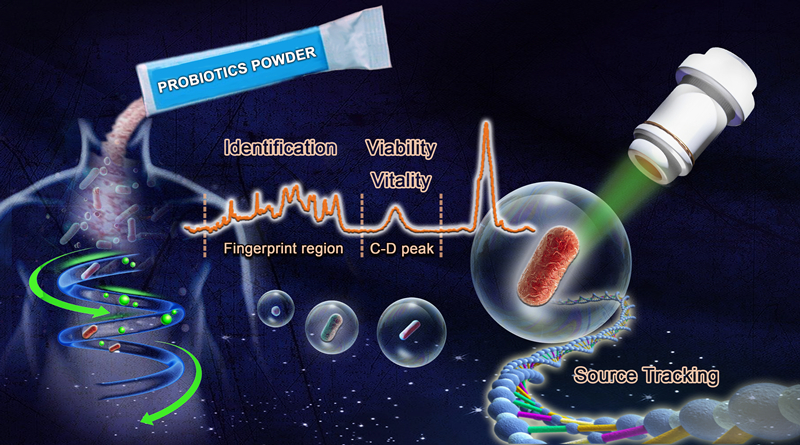
Ramanome-based SCIVVS for the quality assessment of probiotic products (Image by LIU Yang)
Conventional quality assessment processes take more than a week, as technicians must culture bacterial samples in the lab before they can count for the number of live bacteria or retrieve their genetic material for further analysis.
"Instead of requiring several days to culture colonies and individually count in traditional methods, SCIVVS is a streamlined process that can rapidly count live bacteria, identify species of the cell, and test their viability plus their vitality in just five hours, all at single-cell resolution and without culture. In 10 hours, SCIVVS can also track the source of individual cells in the sample from the DNA extracted from just one bacterial cell," said ZHANG Jia, first author of this study and associate professor from Single-Cell Center of QIBEBT.
In addition, the researchers designed SCIVVS to be suited for processing large sample volumes by taking full advantage of the high speed and throughput of Raman microspectroscopy—a label-free imaging technique that reveals the unique fingerprint of different bacteria, and single-cell genome sequencing that produces the genetic blueprints of an individual cell.
"SCIVVS is a new technological and data framework that can transform current practice in quality control, process monitoring and intellectual-property protection of probiotics and other live-cell products," said Prof. XU Jian, co-corresponding author of the study and the head of Single-Cell Center of QIBEBT.
The approach was also quicker and cheaper than traditional methods. "Based on our estimate of experimental duration and consumable costs, SCIVVS takes about five hours and costs $4.39 for a comprehensive quality assessment process," said ZHANG. "It takes about an additional 10 hours and $1.12 to prepare the samples for source tracking. In contrast, traditional methods take up to 11 days and can cost up to about $58."
Next, the researchers plan to further develop and improve the SCIVVS approach, including expanding its abilities to assess other live-cell products, such as cellular immunotherapies or even cancer vaccines.